Developer comes in different strengths the common ones being 10 volume 20 volume 30 volume and 40 volume. State what percentage and volume strength hydrogen peroxide means o.

Bizarre Uses For Hydrogen Peroxide You Will Love Chemistry Cachet
One pint of 3 hydrogen peroxide will release 10 pints of oxygen as it breaks down.

. Chlorine Oxygen Fluorine Hydrogen Peroxide concentrated Nitric Acid. Explain how you would prepare each of the following hydrogen peroxide solute and normal saline solvent irrigation orders1L of 14 strength solution1L 1q 32 fl oz 2pt 960 mL is this correctThe question is asking for an answer in mL. It is the simplest peroxide molecules containing two oxygen atoms covalently bonded to one another and is comme rcially available in aqueous solution over a wide concentration range.
Higher exposures may cause a build-up of fluid in the lungs pulmonary edema a medical emergency with severe shortness of breath. 250 mL hydrogen per. Hydrogen peroxide is sometimes used in the treatment of acne.
The peroxide developers oxidizing potential is denoted as its volume. The books answer is. Hydrogen peroxide has been used as an antiseptic since the 1920s because it kills bacteria cells by destroying their cell walls.
With fewer electrons bacteria cells walls become damaged or even completely break apart. Chemistry Science Organic chemistry BOS 3640. Explain the importance of following manufacturers instructions p.
Some common concentrations include. Its possible to buy peroxide in stronger concentrations but theyre much too strong to use on hair and should be avoided. Super high concentrations of hydrogen peroxide like 50 and 70 and 90 are used for some industrial purposes and as rocket fuel.
Describe two effects of different strengths of hydrogen peroxide on the colouring and lightening process. Explain how you would prepare each of the following solution using liquid stock hydrogen peroxide as the solute and normal saline as the solvent. 3 the typical concentration for household products.
Unfortunately hydrogen peroxides oxidation also destroys healthy skin cells. Download the Android app. Higher exposures may occur from industrial use.
Gee now there is something to think about. The main uses of hydrogen peroxide are in the preparation of other. Are the same strength.
The strength of different oxidizers varies. For example 3 hydrogen peroxide is V10 or 10 volume because it will release 10 times its volume in oxygen. Of course V20 will release twice as much oxygen 20 times its volume.
The higher the strength of peroxide the more oxygen that is produced from it and the more lift you will get. Exposure to hydrogen peroxide can cause irritation of the eyes throat respiratory airway and skin. It acts as a bleaching agent and is also used as a disinfectant.
Explain the uses of hydrogen peroxide when colouring and lightening the hair n. What are the different strengths of hydrogen peroxide. Inhaling Hydrogen Peroxide can irritate the lungs causing coughing andor shortness of breath.
Food grade hydrogen peroxide is composed of 35 hydrogen peroxide and 65 water. Our products are processed and shipped the same day. Food grade hydrogen peroxide does not contain phenol sodium sanate acetanilide and tetrasodium pyrophosphate.
Exposure to Hydrogen Peroxide can cause headache. Designed for use when you simply want to add a tint or color tone to hair of the same lightness. Inhaling Hydrogen Peroxide can irritate the nose and throat.
For household use a solution of 3 is available. The effects of these developer strengths are as follows. Most haircolor formulas today work with a 10 20 or 30 volume developer.
Low exposure may occur from use at home. The chemical formula for hydrogen peroxide is. The higher the percentage of peroxide the more dangerous it is and the more special handling it takes.
Uses of hydrogen peroxide To darken the base colour to lighten the base colour to tone Level 2 Award Certificate Diploma in Hairdressing and Barbering 3002 51. These are stabilizers found in other hydrogen peroxide strengths and these stabilizers could not be ingested. Higher concentrations are 30 for chemical and medical use and up to 70 or anything in.
Concentrated hydrogen peroxide is a very reactive oxygen species and is used as a propellant in rocketry. How the different strengths of hydrogen peroxide affect colouring and lightening the reasons for pre-softening and pre-pigmenting hair effects of temperature on the application and development of colour correction products how to dilute hydrogen peroxide to form different strengths of solutions the pH values of. Answer provided by our tutors.
B 16 fluid ounce of 12 strength for wound care. It is a colourless liquid and is used in aqueous solution for safety reasons. Hydrogen peroxide is the simplest kind of peroxide available oxygen-oxygen single bond.
Hydrogen peroxide is sold in drugstores and grocery stores at a low concentration usually at 3 to 9 percent. A 4 fluid ounce of 14 strength for skin cleansing. Using 1 ounce of liquid stock hydrogen peroxide and 3 ounces of normal saline.
Hydrogen peroxide is a manufactured chemical although small amounts of hydrogen peroxide gas may occur naturally in the air. Ad Get your 100 Certified Food Grade 35 Hydrogen Peroxide delivered to your door. In the laboratory the most commonly seen of the different hydrogen peroxide concentrations is 30.
6 and 20 vol. List the below oxidizers from strongest oxidizing agent to the weakest. Hydrogen peroxide H 2O2 is a weakly acidic colourless li quid miscible with water in all proportions.
Hydrogen peroxide may come in different strengths or concentrations depending on the intended use. It can be used as a disinfectant and as bleach including as a hair lightener. Developer strength is sometimes represented as a percentage.
Describe the different consultation techniques used to identify service objectives q. 10 Volume Peroxide is a standard oxidizing strength for permanent no-lift haircolor. Slightly higher human-use peroxide concentrations may be available in some locations 6 is readily available for instance in the UK.
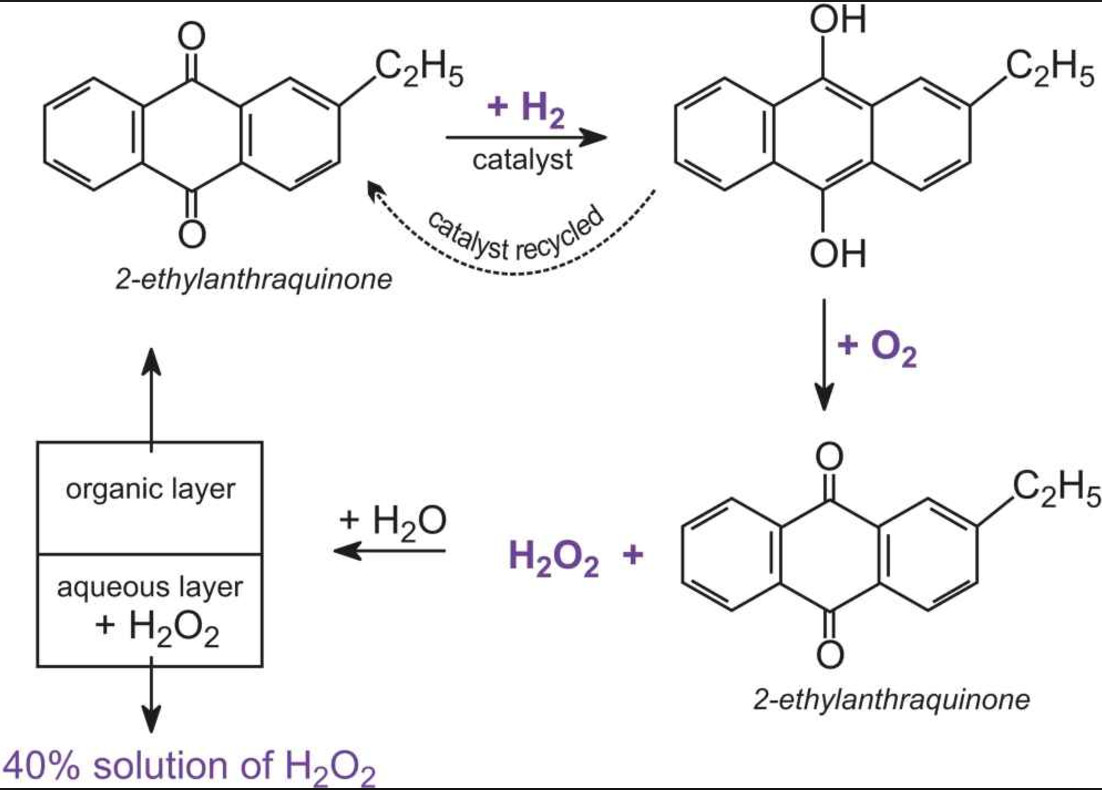
Hydrogen Peroxide Chemistry Class 11 Hydrogen
Hydrogen Peroxide Uses Memozing E Learning Network

Bizarre Uses For Hydrogen Peroxide You Will Love Chemistry Cachet
0 Comments